🧠 New gel offers hope for brain tissue regeneration
Cerebral hemorrhage causes many deaths and disabilities worldwide. Treatment for brain tissue loss has been lacking - but now there may have been a breakthrough.
Share this story!
Brain bleeding and brain cancer cause many deaths and disabilities worldwide. The brain can be easily harmed when blood supply is lost, leading to the brain getting smaller and damaged. Unlike other body parts that can heal on their own, brain tissue loss can't be fixed.
We need a good way to help the brain heal. Unfortunately, there isn't any proven treatment method yet – but now there might have been a breakthrough.
A breakthrough in brain tissue regeneration
Researchers at Hokkaido University have developed synthetic hydrogels that provide an effective scaffold for neuronal tissue growth in areas of brain damage. This discovery could offer a new approach for treating life-threatening conditions caused by brain hemorrhage and brain cancer.
Hydrogel optimization
Scientists led by Satoshi Tanikawa have found that a neutral hydrogel with a 1:1 mixture of anionic and cationic monomers (C1A1) generated the most suitable scaffold for attachment, growth, and differentiation of neural stem cells (NSCs).
They used cryogelation to create pores in the C1A1 hydrogels, allowing for a 3D culture of cells in the porous structure. The hydrogel's stiffness was also adjusted to match that of brain tissue, a crucial factor in directing stem cell differentiation.
Testing in a mouse model
After optimizing the hydrogel formulation, the researchers tested its potential for in vivo brain tissue reconstruction using a mouse model. The porous hydrogel was implanted into a cylindrical cavity created in the mouse brain.
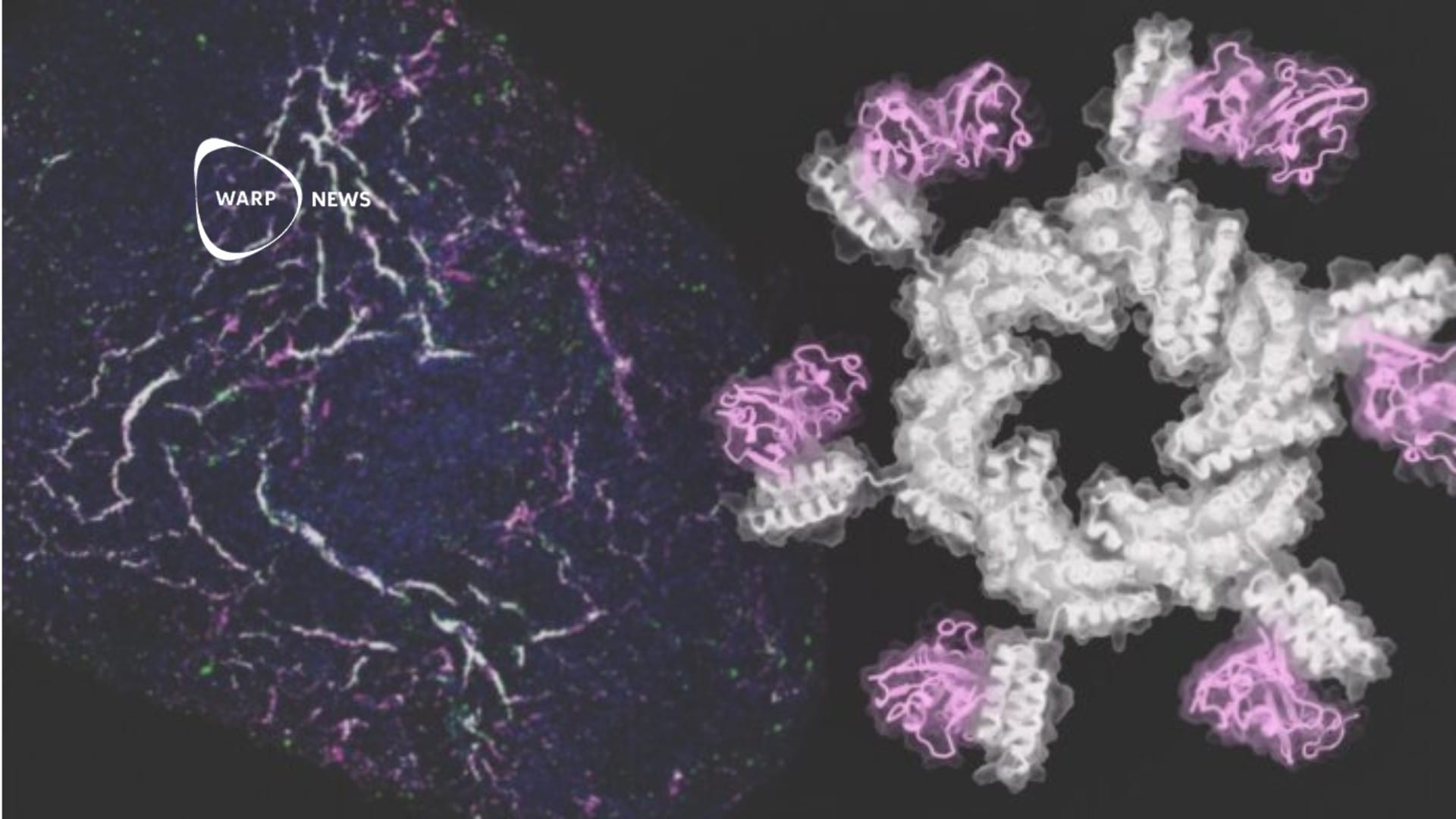
On day 56 after implantation, the boundary between the brain tissue and the hydrogel was obscured, while cavities clearly remained in control brains. Immune cells and astrocytes from surrounding brain tissue had infiltrated into the pores of the implanted hydrogel.
Encouraging vascularization and cell survival
To induce vascularization, the C1A1 hydrogel was immersed in vascular endothelial growth factor (VEGF) before implantation. This step led to the formation of blood vessels inside the hydrogel two to three weeks after implantation. Fluorescently labeled NSCs were injected into the implanted hydrogel in the mouse brain, and forty days later, fluorescence images showed viable stem cells with a high survival rate. Some of the NSCs had differentiated into new glial or neuronal cells, which had migrated into surrounding host brain tissue.
A promising two-step method
The researchers emphasize the importance of the two-step process, as implanting the hydrogel and NSCs simultaneously proved unsuccessful. The mice implanted with hydrogels showed no behavioral abnormalities or deaths.
Senior author Shinya Tanaka states that they are currently working to prove functional recovery with their method in a mouse model of brain damage, a critical evaluation for future possible clinical applications.
By becoming a premium supporter, you help in the creation and sharing of fact-based optimistic news all over the world.