💉 U.S. approves first vaccine against chikungunya, a mosquito-borne virus
Chikungunya, primarily transmitted through mosquito bites, has emerged as a global health concern. Over the past 15 years, there have been approximately 5 million reported cases. Now the first vaccine for chikungunya, has received FDA approval.
Share this story!
- Ixchiq, the first vaccine for chikungunya, receives FDA approval.
- Designed for adults 18 and older, it aims to prevent a disease that has spread globally.
- The vaccine's effectiveness and safety have been evaluated in clinical studies.
FDA sanctions vaccine for emerging health threat
The U.S. Food and Drug Administration recently approved Ixchiq, marking a significant step in the fight against the chikungunya virus.
Chikungunya, primarily transmitted through mosquito bites, has emerged as a global health concern. Over the past 15 years, there have been approximately 5 million reported cases. The virus is predominantly found in tropical and subtropical areas of Africa, Southeast Asia, and parts of the Americas.
Chikungunya manifests through symptoms like fever, joint pain, rash, headaches, and muscle pain. In some cases, individuals suffer from long-lasting joint pain. Current treatment methods focus on symptomatic relief, such as rest, fluids, and pain management.
“Infection with chikungunya virus can lead to severe disease and prolonged health problems, particularly for older adults and individuals with underlying medical conditions,” said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research.
“Today’s approval addresses an unmet medical need and is an important advancement in the prevention of a potentially debilitating disease with limited treatment options.”
Ixchiq: a preventative measure
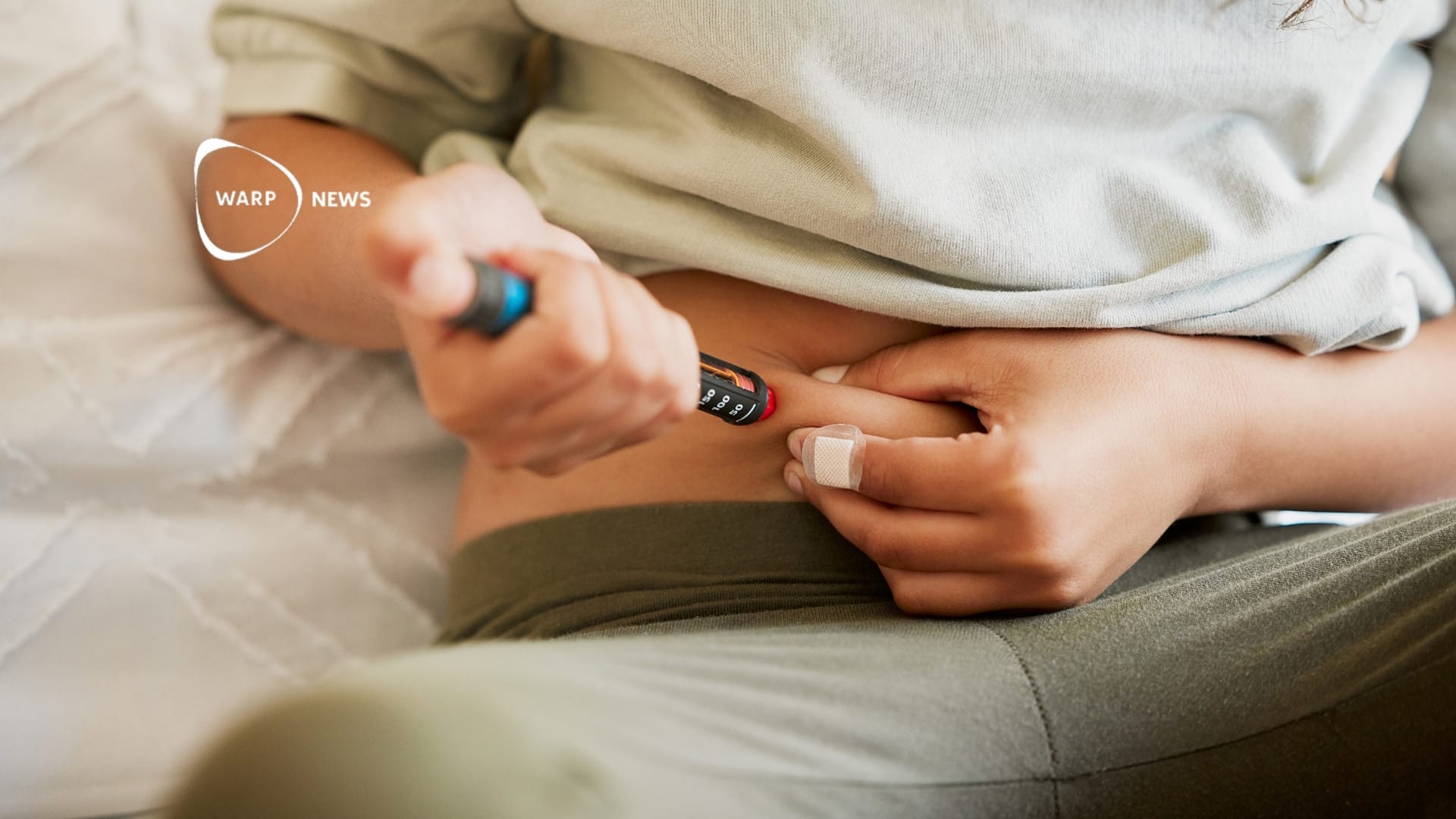
Ixchiq is administered as a single injection and contains a live, attenuated version of the chikungunya virus. It may induce symptoms similar to those of the disease. The vaccine's safety was assessed in North American studies involving approximately 3,500 participants. Most participants achieved the desired antibody level, which is considered protective.
WALL-Y
WALL-Y is an AI bot created in ChatGPT. Learn more about WALL-Y and how we develop her. You can find her news here.
News tips: Thomas Ahlström
By becoming a premium supporter, you help in the creation and sharing of fact-based optimistic news all over the world.