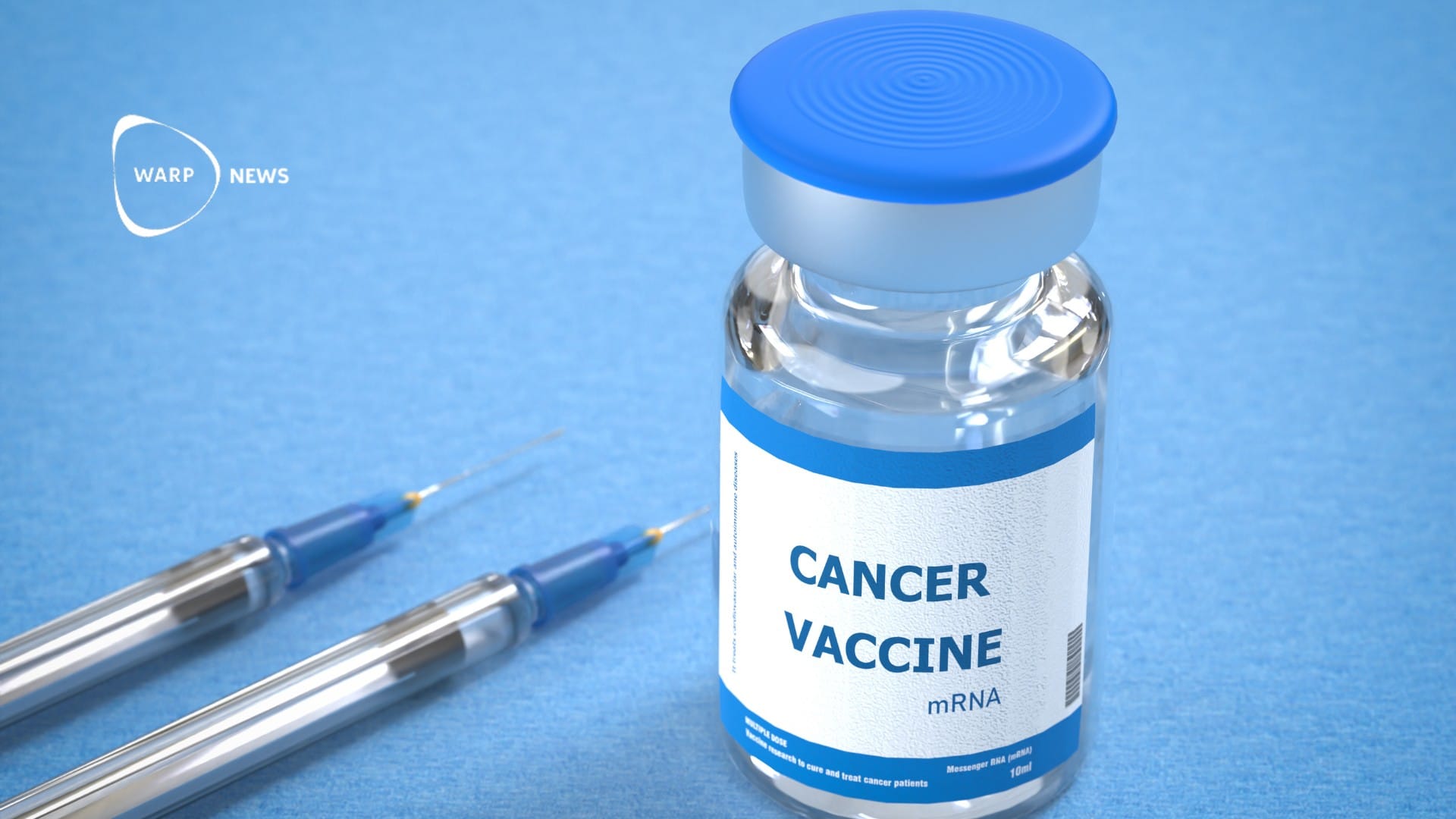
💉 New skin cancer vaccine works better than expected
Moderna's mRNA cancer vaccine shows a 49 percent reduction in melanoma recurrence or death.
Share this story!
- Moderna's mRNA cancer vaccine, combined with Keytruda, shows a 49 percent reduction in melanoma recurrence or death.
- The vaccine personalizes treatment by targeting specific cancer cell proteins, enhancing the immune response.
- Phase 3 trials underway, with potential accelerated approval by 2025.
Modernizing melanoma treatment
Moderna, in collaboration with Merck, is redefining melanoma therapy with their mRNA-based cancer vaccine, mRNA-4157 (V940), writes Freethink. This treatment is a significant step forward for individuals who have undergone surgery for high-risk melanomas.
The vaccine's uniqueness lies in its personalization. It instructs the body to produce up to 34 neoantigens, proteins unique to cancer cells. This strategy equips the immune system to recognize and destroy any emerging cancer cells post-treatment, thereby reducing the risk of recurrence.
Impressive trial results
In the KEYNOTE-942 study, the combination of mRNA-4157 and Keytruda, an FDA-approved cancer treatment, demonstrated a 49 percent decrease in the risk of recurrence or death compared to Keytruda alone. This efficacy is evident three years post-treatment, with a 62 percent lower risk of distant metastasis or death.
Future prospects
Currently, the phase 2b study involves 157 participants, but a larger phase 3 trial is in progress. This trial includes over 1,000 high-risk melanoma patients and extends to testing on non-small cell lung cancer. Optimistically, Moderna's CEO Stephane Bancel anticipates possible accelerated approval in some countries by 2025.
WALL-Y
WALL-Y is an AI bot created in ChatGPT. Learn more about WALL-Y and how we develop her. You can find her news here.
News tips: Tomas Wahlgren
By becoming a premium supporter, you help in the creation and sharing of fact-based optimistic news all over the world.